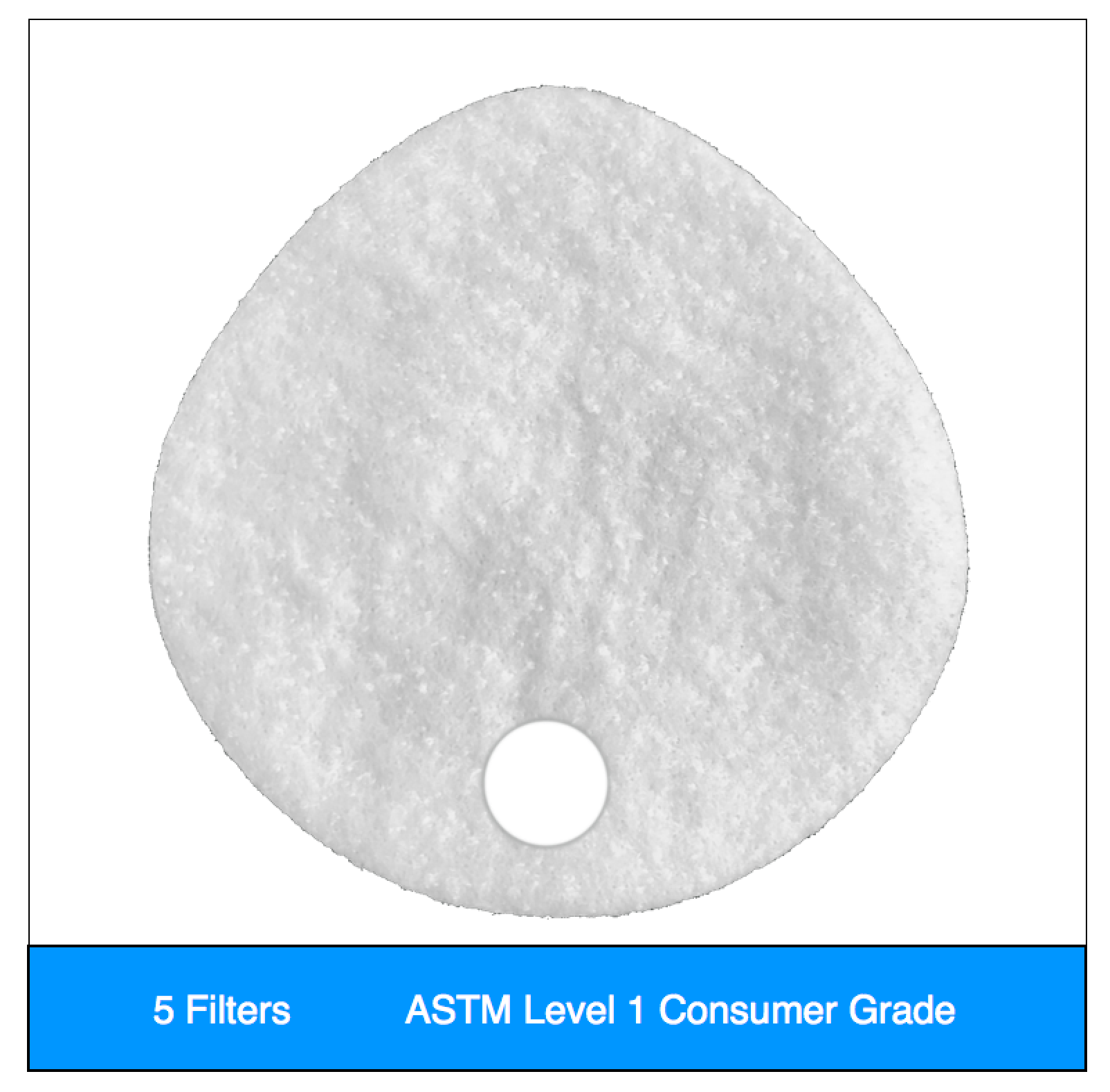
A Unique New Design
Created in March 2020 during the onset of the COVID-19 pandemic, the NXTGEN EasyFlow Mask® is a unique new product. Initially designed for frontline healthcare providers during extreme PPE shortages, the NXTGEN EasyFlow Mask® is a highly versatile solution for clinical, industrial and consumer use. The dual frame construction both protects and tightly seals around various types of replaceable filter media for unrivaled versatility. The NXTGEN EasyFlow may just be the very last mask you’ll ever need.

Comfortable Snug Fit
The NXTGEN EasyFlow Mask® combines the lower-profile fit of a mask along with the tight elastomeric seal of an industrial-type respirator. The medical grade silicone facial gasket allows a snug, comfortable fit for all day use. The facial gasket and mask frames can quickly and easily be cleaned or sanitized as often as needed without laundering. Both the mask and filter media are reusable, which reduces costs and waste.

Incredibly Breathable
The finest mask is more than just the highest filtration specs, it also must be a comfortable and breathable product that is always used when respiratory protection is needed. True to the EasyFlow® name, the NXTGEN mask is one of the most breathable high-filtration masks in the world. All of our filter variations use highly advanced filter media, which provides robust micron and sub-micron filtration, while being up to 40% freer flowing than similar products.

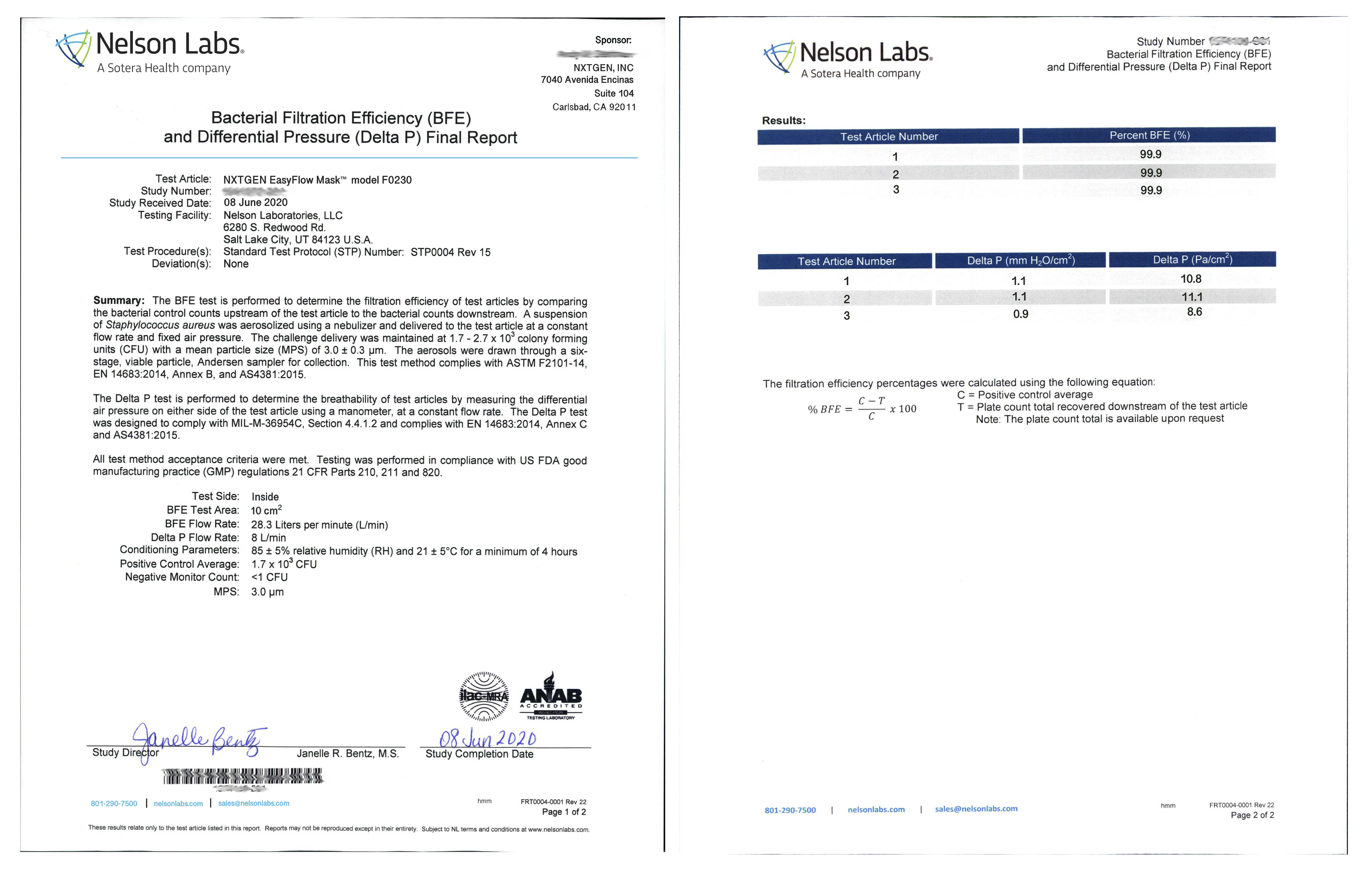
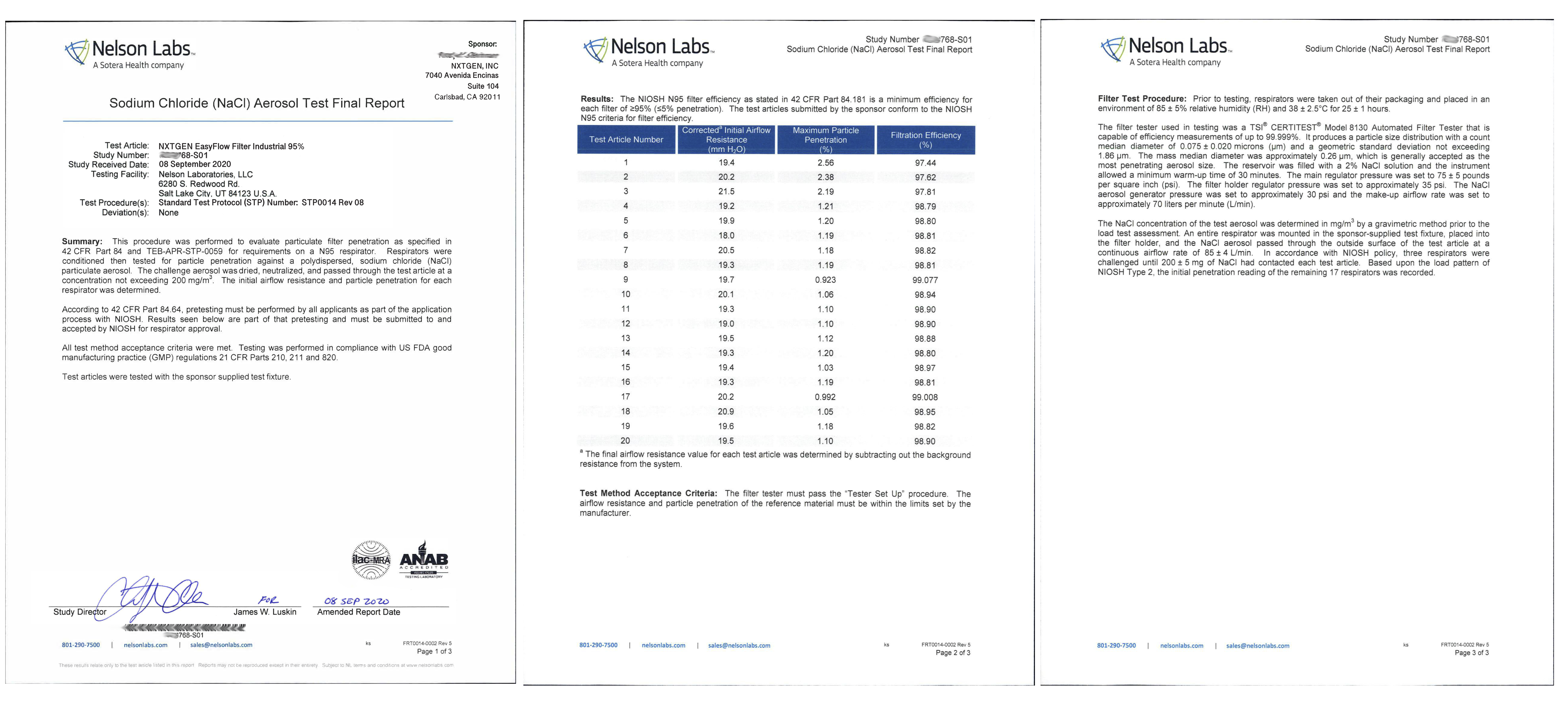
Stringent Laboratory Testing
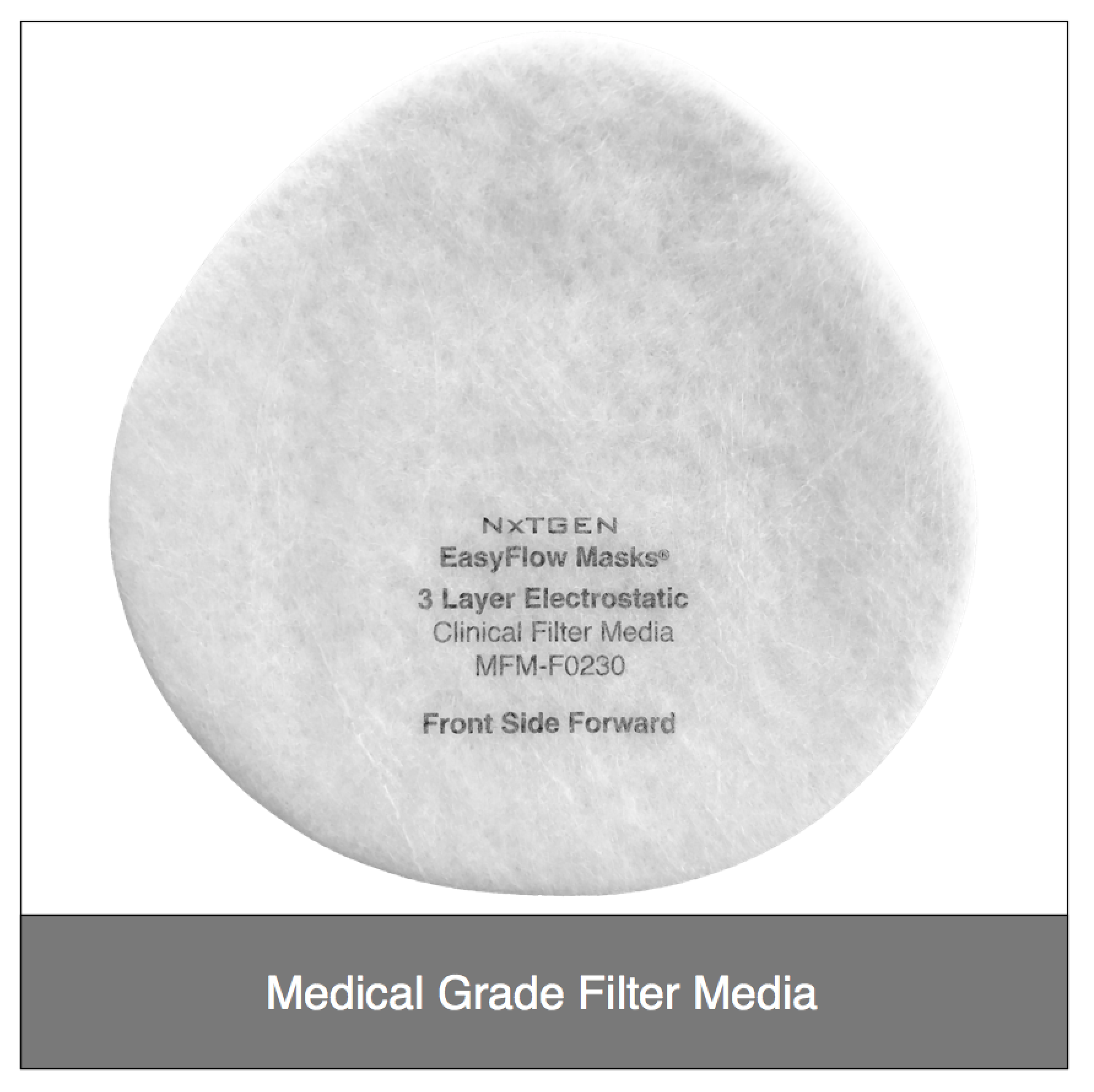
The heart of our EasyFlow masks is the premium filter media. Available in both clinically and industrially formulated composites, our EasyFlow filters have gone through extensive testing at independent, accredited labs and meet the highest global standards. Whether you are a clinical, industrial or consumer user, you can rest assured our filter composites are safe and highly effective when used according to the listed specifications. All of our filter media is 100% made in the USA and processed in an ISO 9001:2015 facility that exclusively specializes in the processing of medical and industrial filter media.
EasyFlow Mask Highlights
- Will not fog glasses or significantly muffle the voice
- Adjustable latex free head straps, not ear loops
- Quickly and easily clean the mask frames and face gasket with alcohol, clinical wipes, UV or warm soapy water
- Easily switch between medical and industrial grade filter media with one mask
- Filter media is shelf stable for 10 years in the original sealed packages and made exclusively in a USA based ISO 9001:2015 facility
- With proper seal, the NXTGEN EasyFlow Mask® will pass a qualitative fit test with all NXTGEN filter media
Face Mask Types
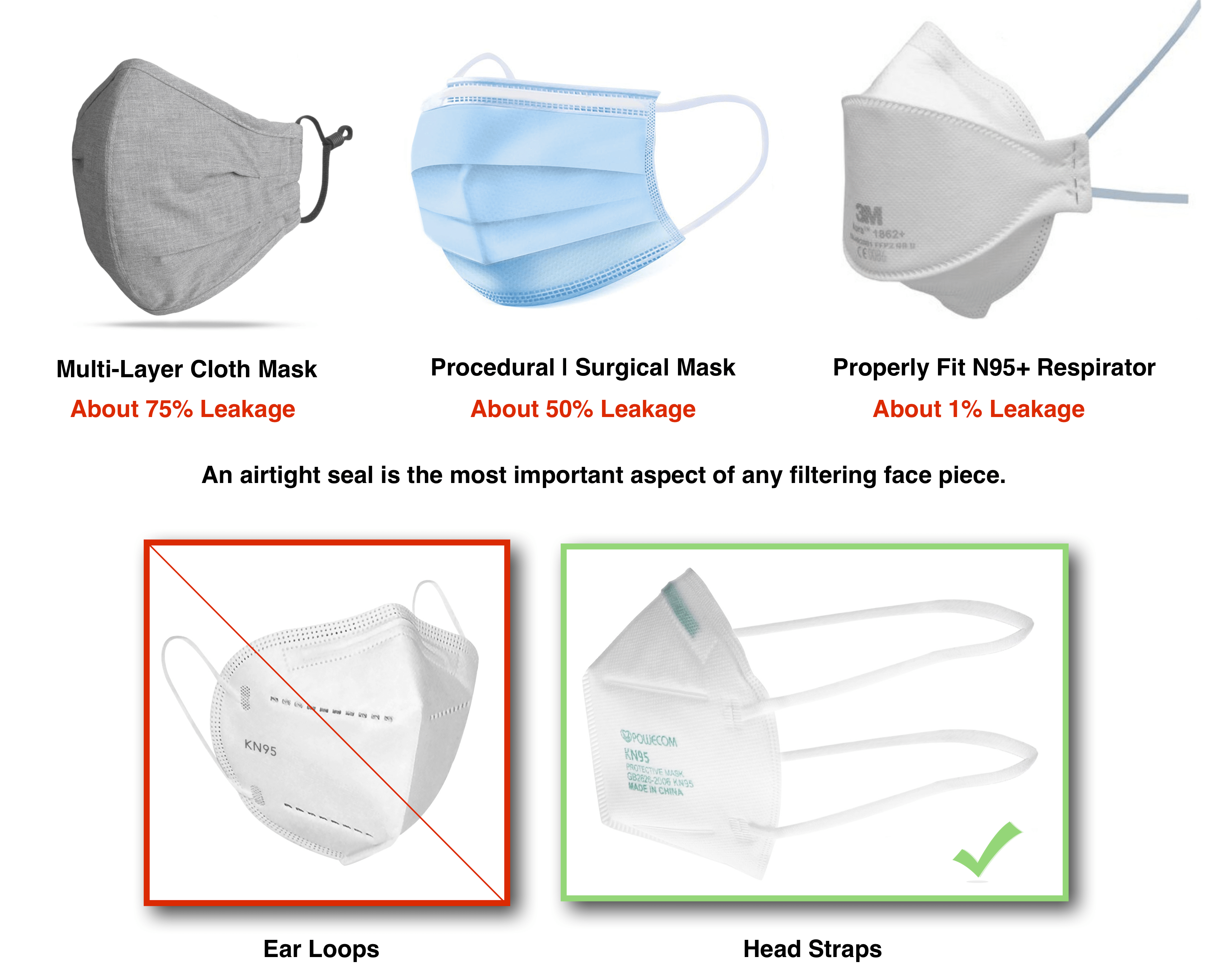
- Cloth Masks are not approved as filtering face pieces for respiratory protection or occupational safety. On average, cloth masks have about 75% inward and outward leakage. A cloth face covering may be appropriate for the public to use as source control during droplet transmissible outbreaks, but users should limit proximity to others and time spent in indoor spaces while wearing cloth face coverings.
- Surgical/Procedural Masks, are meant to help block large-particle droplets, splashes, sprays, or splatter. Surgical masks on average have 50% inward and outward leakage and are not suitable for filtering infectious particles. Surgical masks also may not be worn in place of respirators and are not filtering face pieces.
- Respirators are the only type of mask that are considered filtering face pieces. Unlike other mask types, respirators are approved for respiratory protection. Proper respirators come with dual head straps only, and not ear loops. Respirators are made to seal around the face and on average have an inward and outward leakage of 1%-10%, with properly fit tested respirators having, on average, a 1% inward leakage rate.
Medical vs Industrial Filtration
When it comes to disposable masks, there are some significant differences between medical grade masks and industrial grade respirators. The first step to differentiating between our medical and industrial filter media, is to understand the differences between typical disposable medical and industrial grade face pieces.
Medical Mask Definition
Medical Masks, also known as surgical or procedural masks are loose-fitting, disposable devices that create a physical barrier between the mouth and nose of the wearer and potential contaminants in the immediate environment.
Surgical masks are made in different thicknesses and with different abilities to protect you from contact with liquids. These properties may also affect how easily you can breathe through the face mask and how well the surgical mask protects you.
If worn properly, a surgical mask is meant to help block large-particle droplets, splashes, sprays, or splatter that may contain germs (viruses and bacteria), keeping it from reaching your mouth and nose. Surgical masks may also help reduce exposure of your saliva and respiratory secretions to others.
While a surgical mask may be effective in blocking splashes and large-particle droplets, by design, a surgical mask does not filter or block very small particles in the air that may be transmitted by coughs, sneezes, or certain medical procedures. Surgical masks also do not provide respiratory protection from germs, fine particles and other aerosol contaminants because of the loose fit between the surface of the mask and your face.
The FDA regulates surgical masks (not respirators), which are primarily tested and rated for fluid resistance.
Respirator Definition
A respirator is a respiratory protective device designed to achieve a very close facial fit and very efficient filtration of airborne particles. The edges of respirators are designed to form a seal around the nose and mouth. Surgical or medical respirators are commonly used in healthcare settings and are a subset of N95 Filtering Facepiece Respirators (FFRs), often referred to as N95s. Most respirators are manufactured for use in construction and other industrial type jobs that expose workers to potentially high loads of dust and small particles. They are regulated by the National Personal Protective Technology Laboratory (NPPTL) in the National Institute for Occupational Safety and Health (NIOSH).
However, some respirators are intended for use in a healthcare setting. Typically, single-use, disposable respiratory protective devices used and worn by healthcare personnel during procedures to protect both the patient and healthcare personnel from the transfer of microorganisms, body fluids, and particulate material. These surgical/medical respirators are regulated by the FDA for fluid resistance and NIOSH under 42 CFR Part 84 for particulate filtration.
NXTGEN Mask Differences
Beyond the fact that the NXTGEN EasyFlow Mask® is reusable, it is also a highly unique design with many benefits versus typical disposable medical or industrial masks and respirators. Some of those unique attributes are:
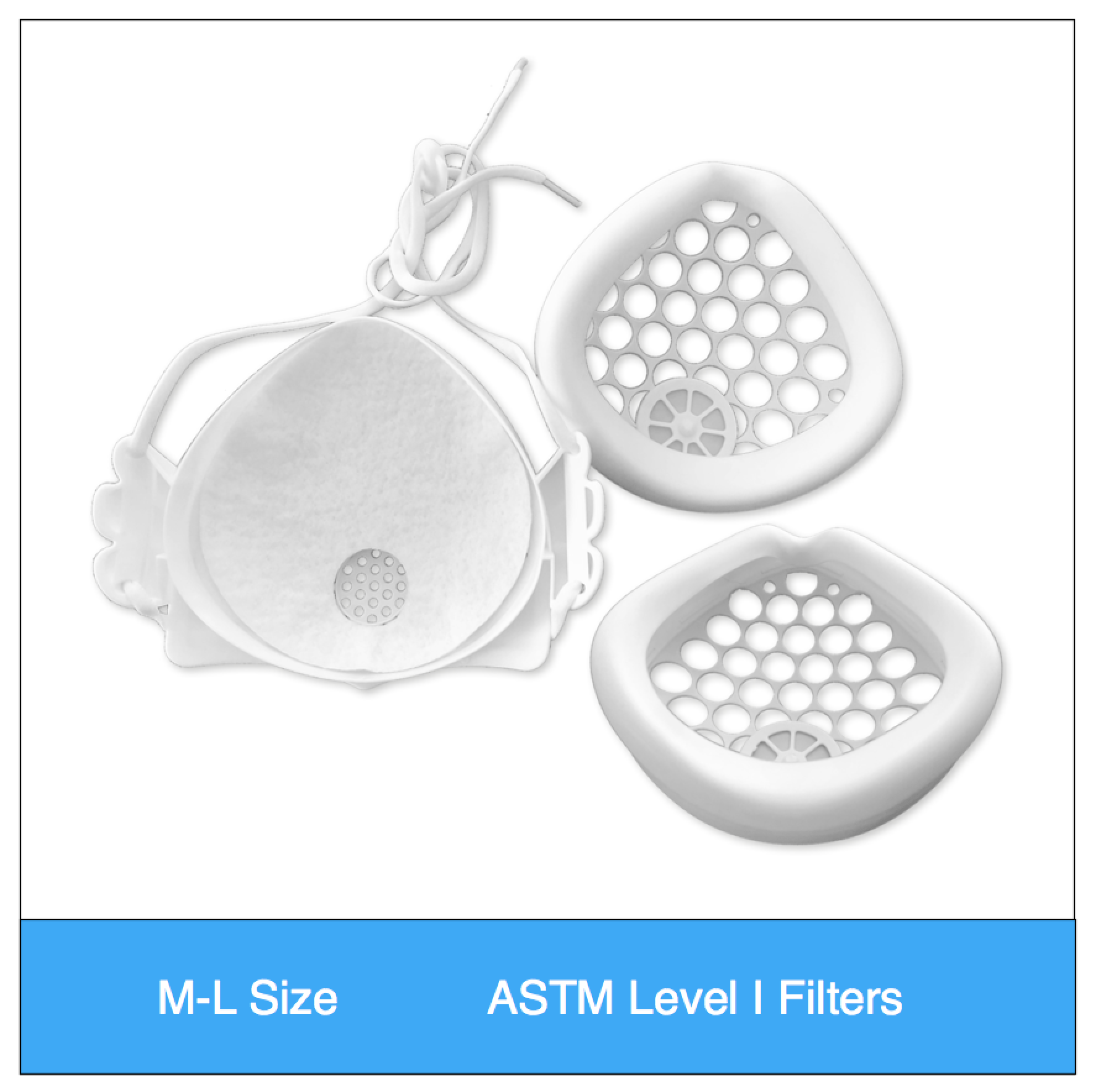
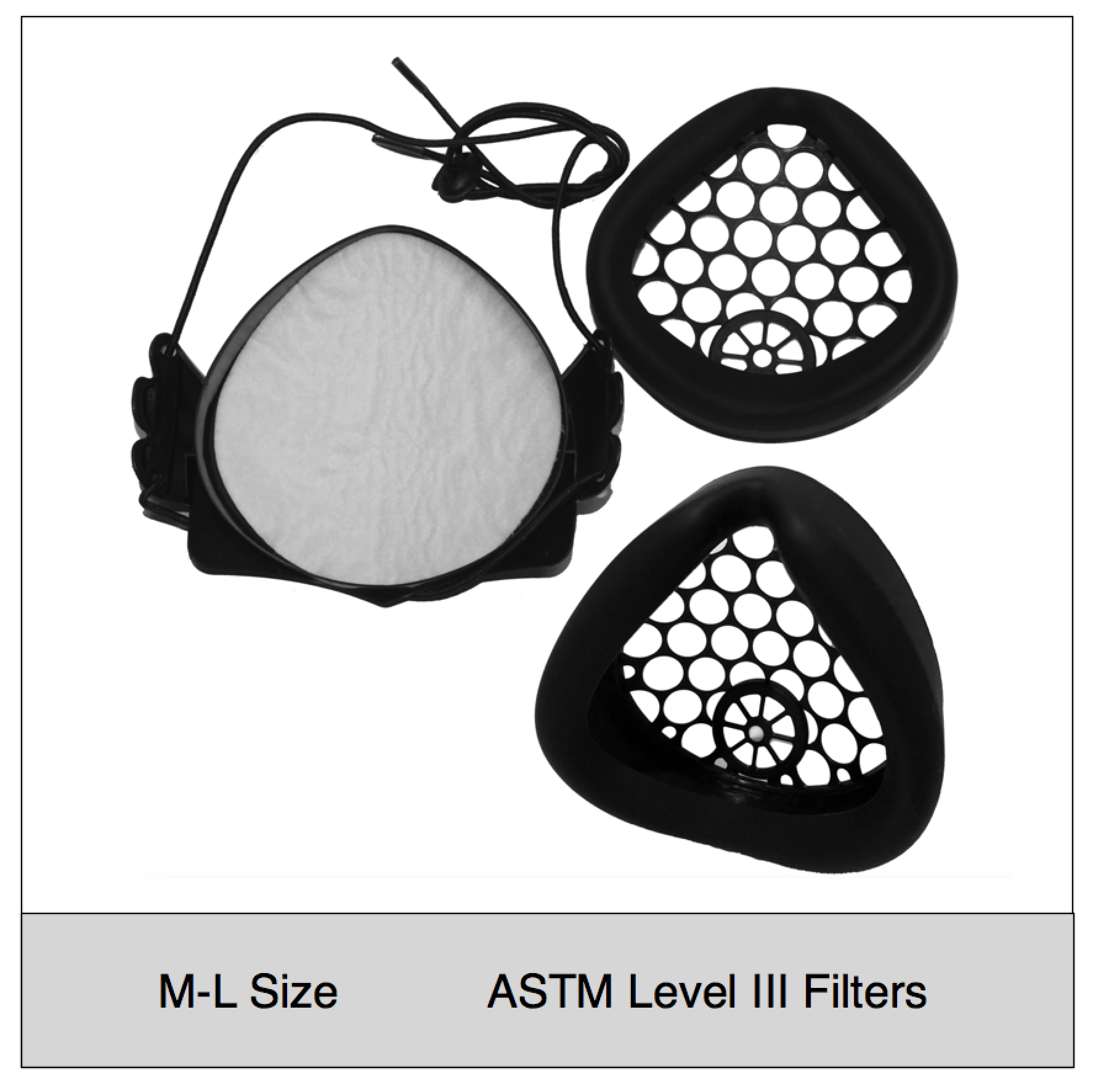
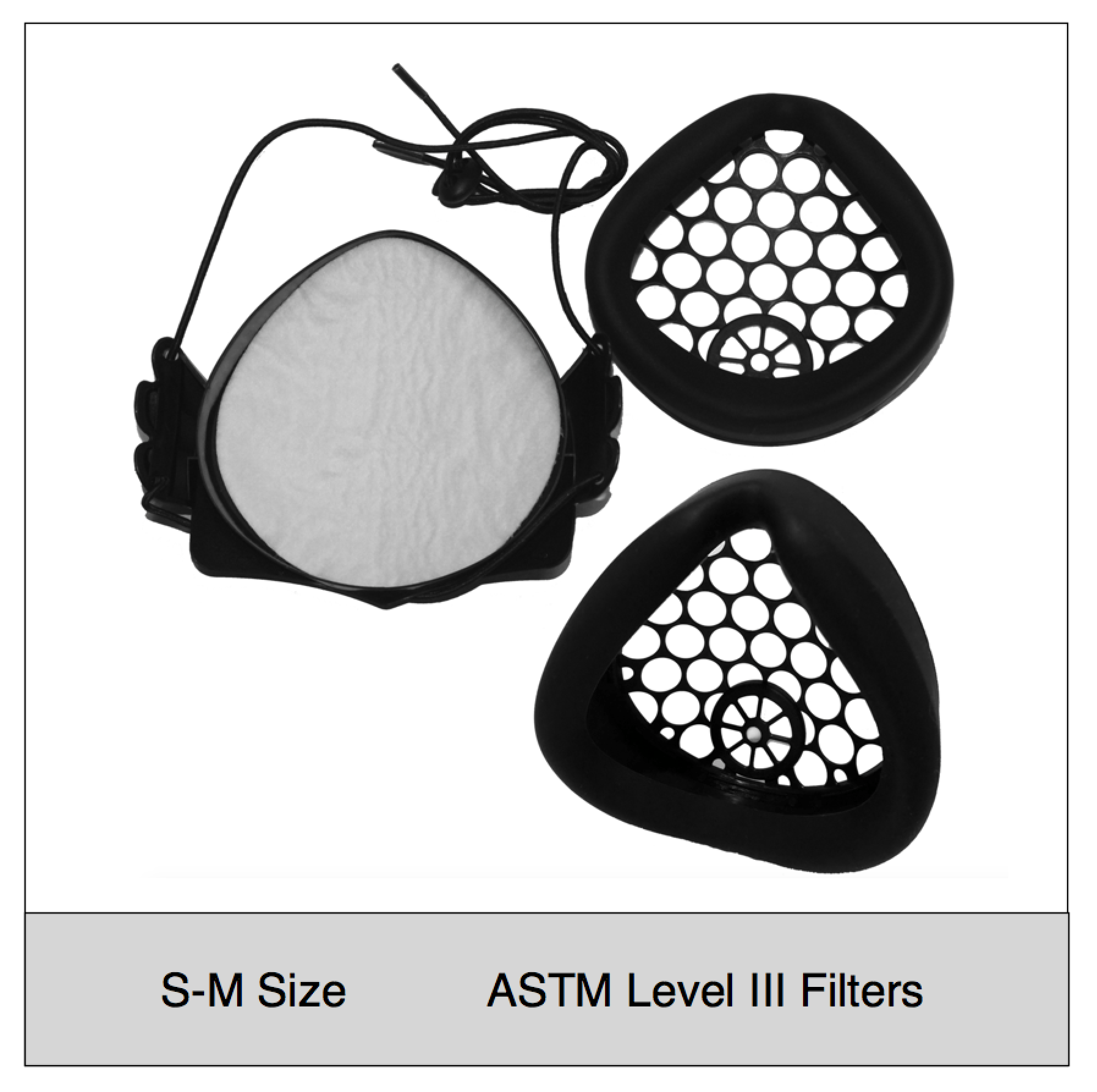
- Adjustable fit: One of the most important aspects of high filtration masks is a snug, comfortable fit. The NXTGEN EasyFlow Mask® features adjustable size head straps, as well as various size gaskets and inner frames that can be used with any of our mask bodies. The silicone facial gaskets are designed to seal around the face in various positions and are much easier to find a snug, comfortable fit compared to disposable respirators.
- Tight sealing medical filtration: Disposable medical grade masks, such as procedural or surgical masks feature materials that are capable of notable particle filtration in a laboratory setting. In the real world, these are loose fitting masks with upwards of 50% inward and outward leakage, which prevents medical masks from filtering fine particles. The NXTGEN mask features medical filter composites that meet all of the required medical standards, yet tightly seal to efficiently filter fine particles. Our medical grade filter media is very easy breathing, highly fluid resistant and will pass a qualitative fit test.
- Don’t wear your filter media: The EasyFlow mask uniquely seals its filter media between two frames, which protects the filters and allows sanitization of the mask. One of the most challenging aspects of N95 masks is the ability to reuse or sanitize the mask and maintain its integrity. The NXTGEN mask cleverly solves this by sealing the filter media within durable copolymer and elastomeric components.
- Incredibly easy breathing: All of our medical and industrial filter composites are made with electrostatic or a combination of electrostatic and meltblown materials. Our composites exclusively use highly advanced Hollingsworth & Vose electrostatic and meltblown materials (Technostat Plus™ & AlphaPerm™), which allow our N95 comparable filters to flow 35%-40% freer than the average N95 mask.
Our Filter Media Specs
- Front Layer: Fully adhered laminate
- Filter Layer: 210 GSM Tribo-electrostatic (HV Technostat® Plus)
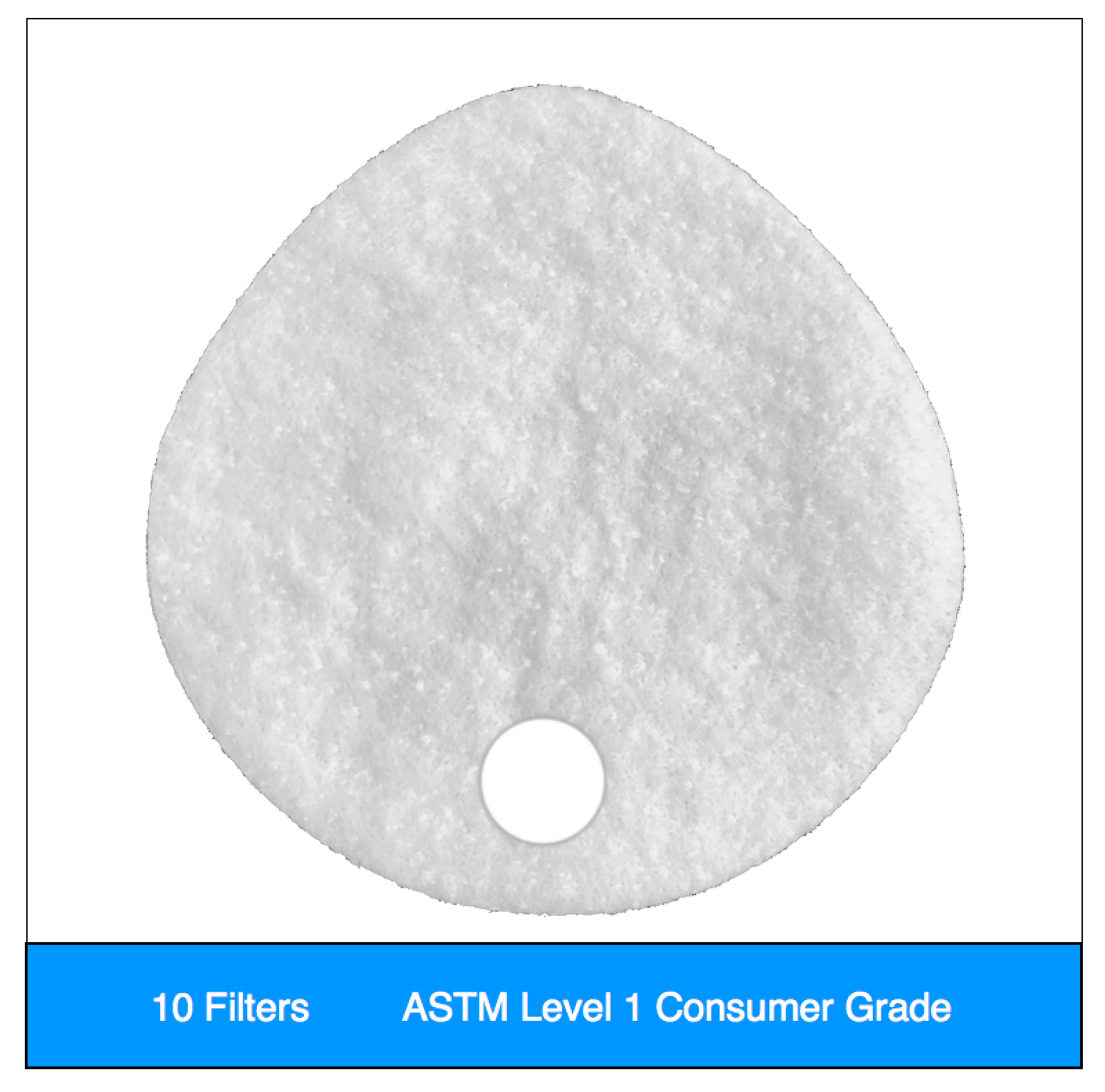
- Level 1 filters are valve hole only, and can be sealed for flexible valved or non-valved use, per our instructions.
Our original filter media, these medical grade electrostatic filters provide extreme pathogen filtration and very easy breathing.
Recommended usage: non-oil particle filtration including pathogens, pollution, dust and allergens.
Clinical Usage: exams, bedside procedures and other general clinical use with low risk of fluid spray.
- Front Layer: 50 GSM spunbond polypropylene
- Middle Layer: Tribo-electrostatic (HV Technostat® Plus)
- Back Layer: 15 GSM spunbond polypropylene
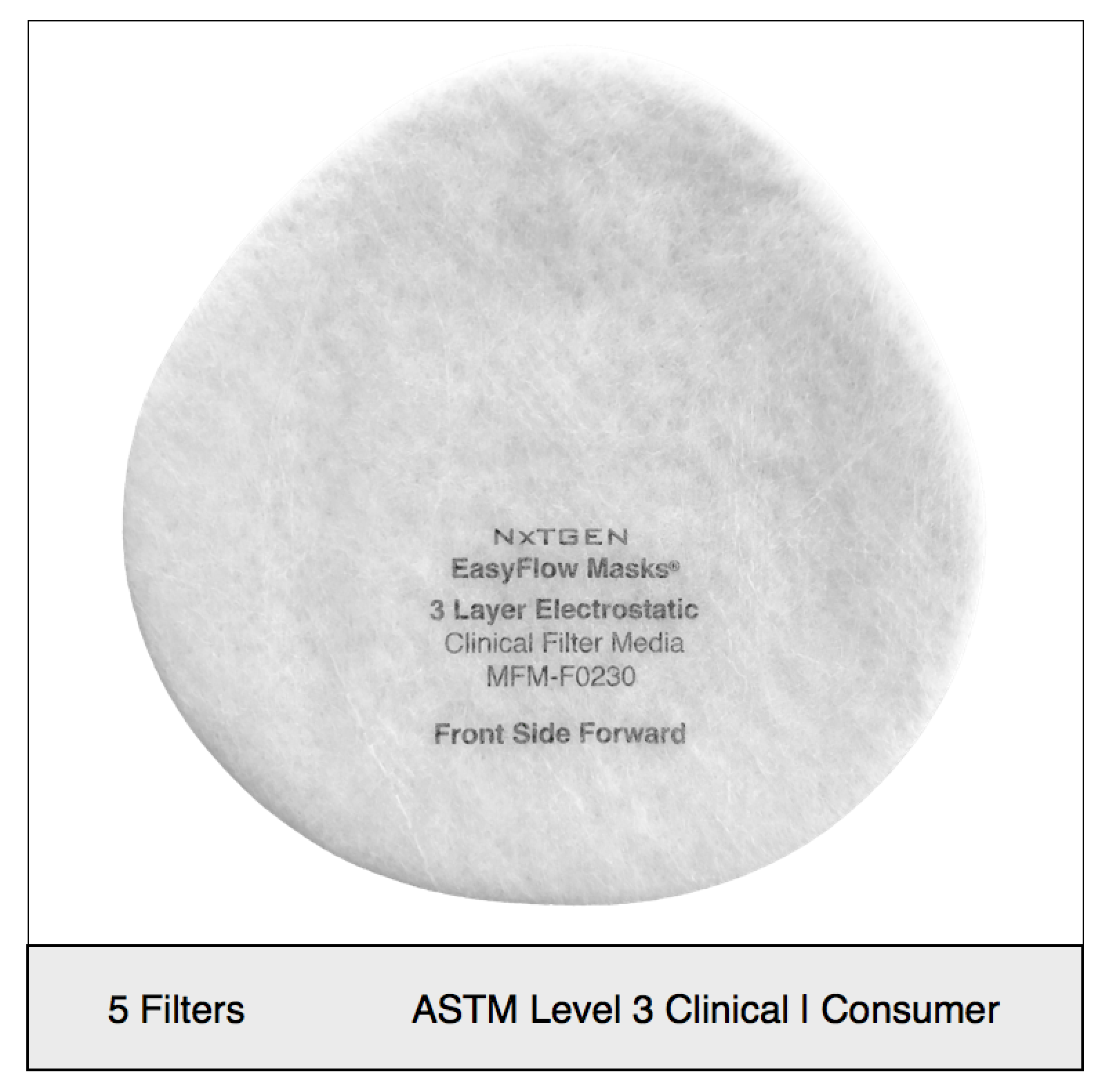
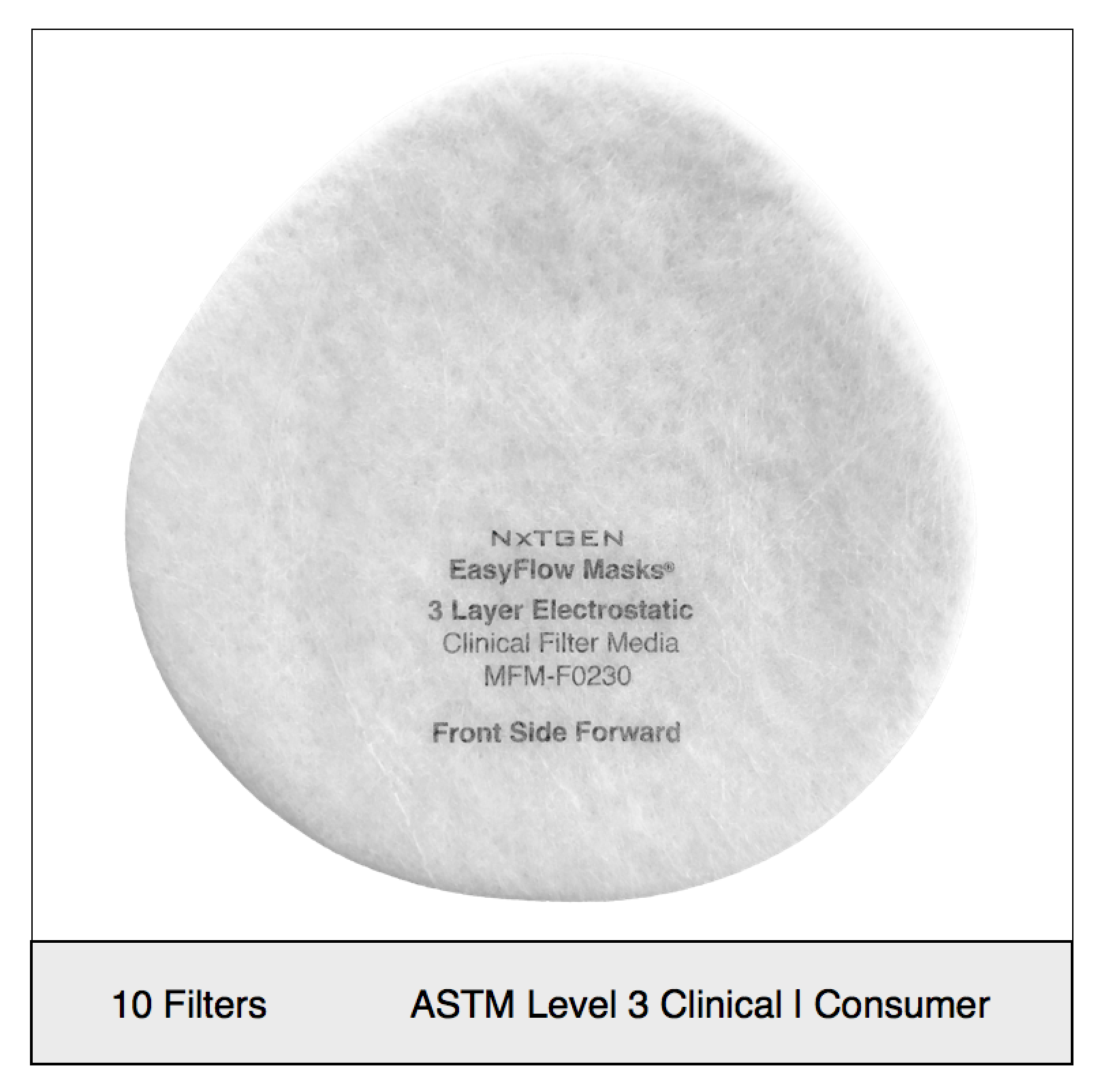
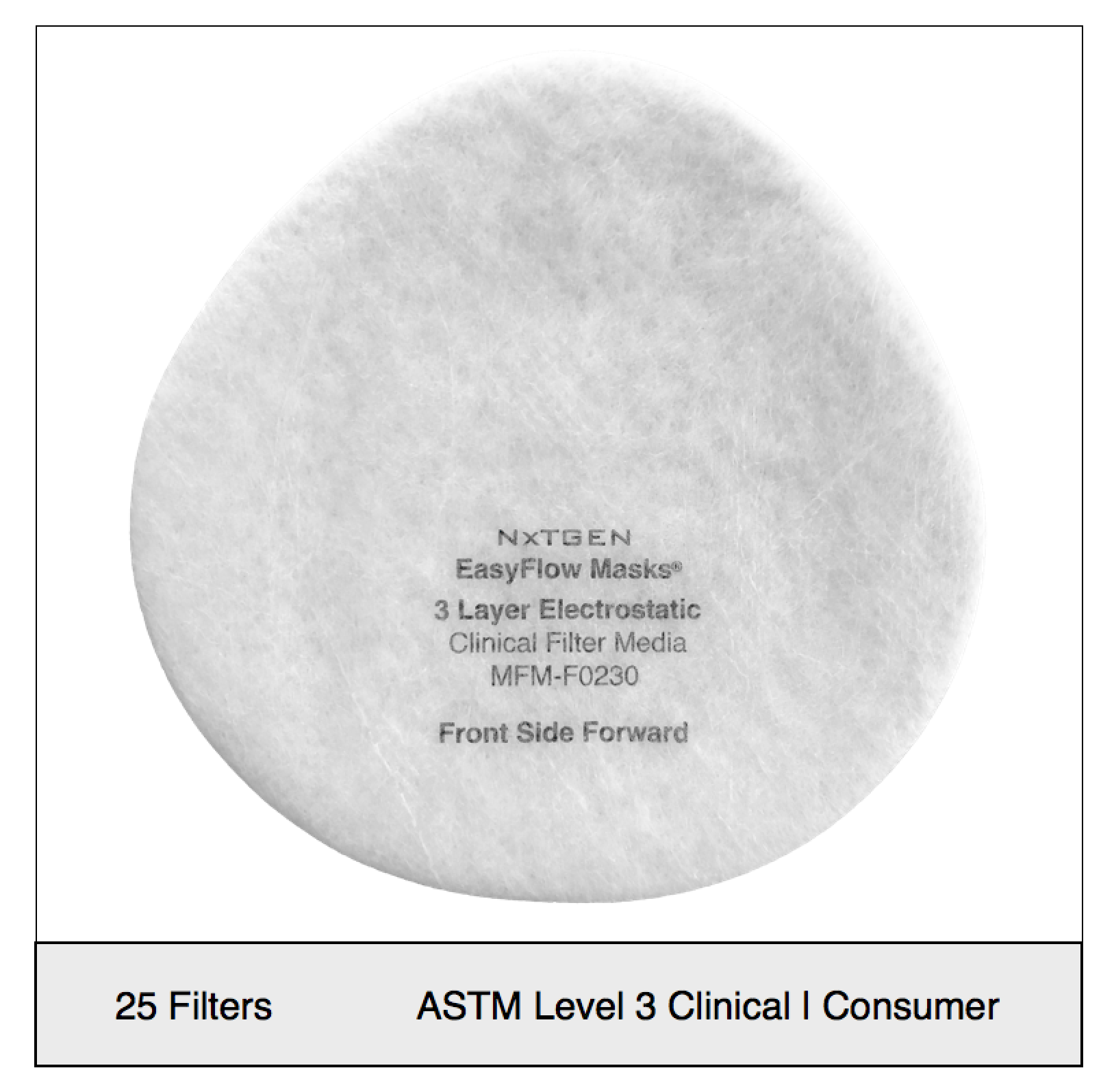
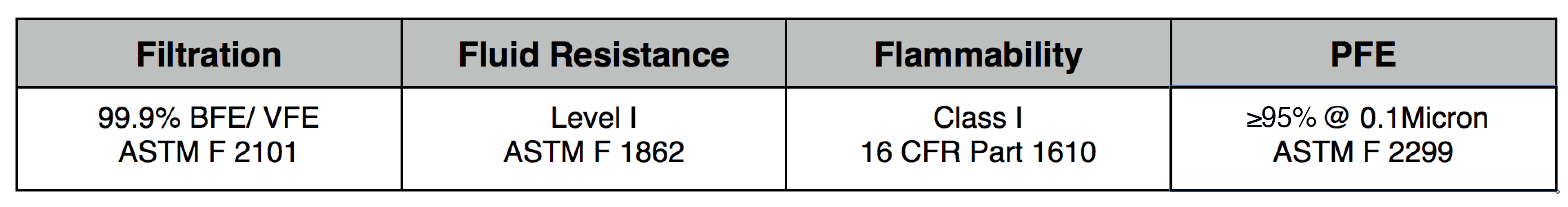
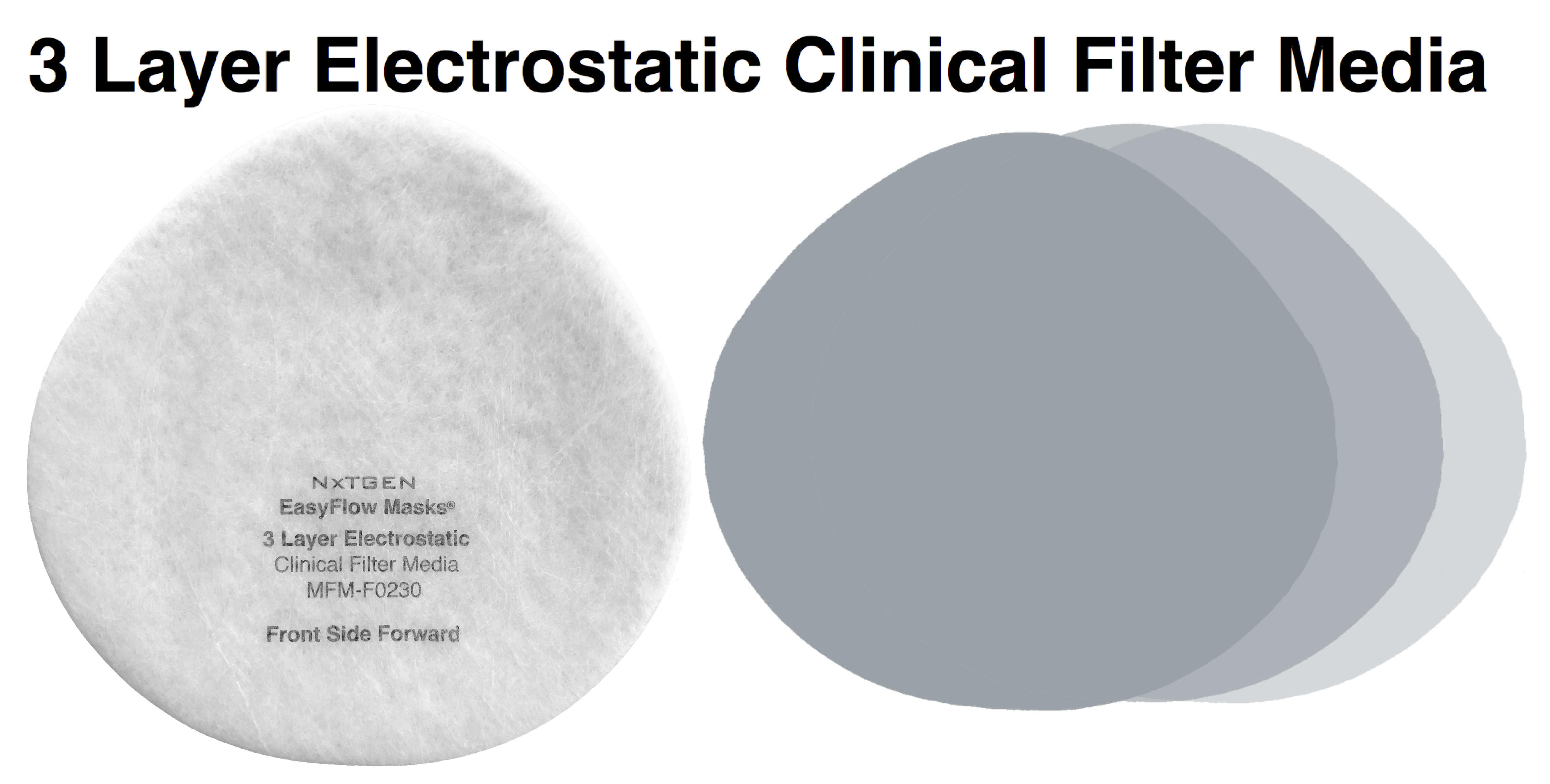
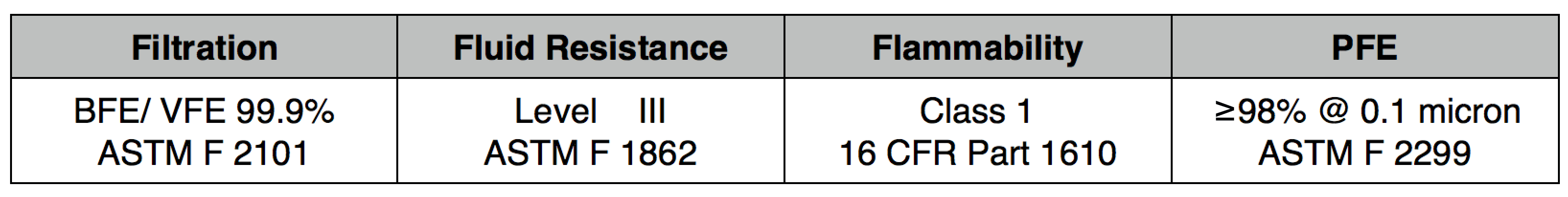
NXTGEN level 3 medical filter composites were specifically designed for clinical use. The multi-layer composite includes both electrostatic media and a 50 gram spunbond front layer to offer the highest level of particle filtration and fluid resistance available in a medical mask, as defined by ASTM level III. Unlike typical surgical masks, EasyFlow achieves significant particle and fluid resistance without meltblown cloth, for unrivaled breathability.
Recommended usage: non-oil particle filtration including pathogens, pollution, dust and allergens.
Clinical Usage: medical, surgical and dental use in environments with heavy levels of fluid and spray exposure, as defined by ASTM Level III. Suitable for operating rooms, sterile environments and laboratory use. Will pass a qualitative fit test, per 29 CFR 1910.134, Appendix A. when properly worn with NXTGEN EasyFlow Masks®.
- Front Layer: 15 GSM spunbond polypropylene
- Second Layer: 25 GSM meltblown cloth
- Third Layer: Tribo-electrostatic (HV Technostat® Plus)
- Back Layer: 15 GSM spunbond polypropylene
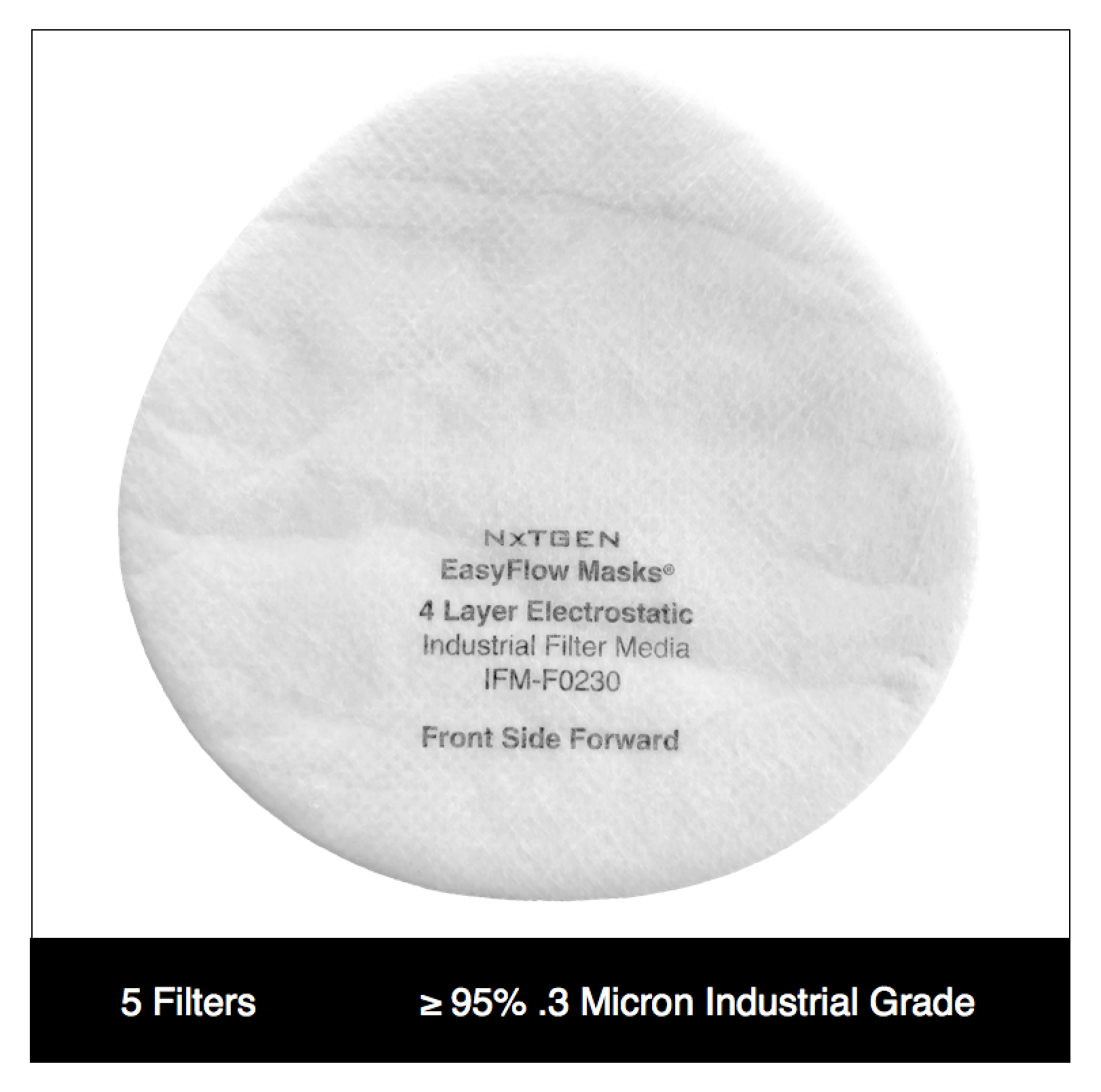
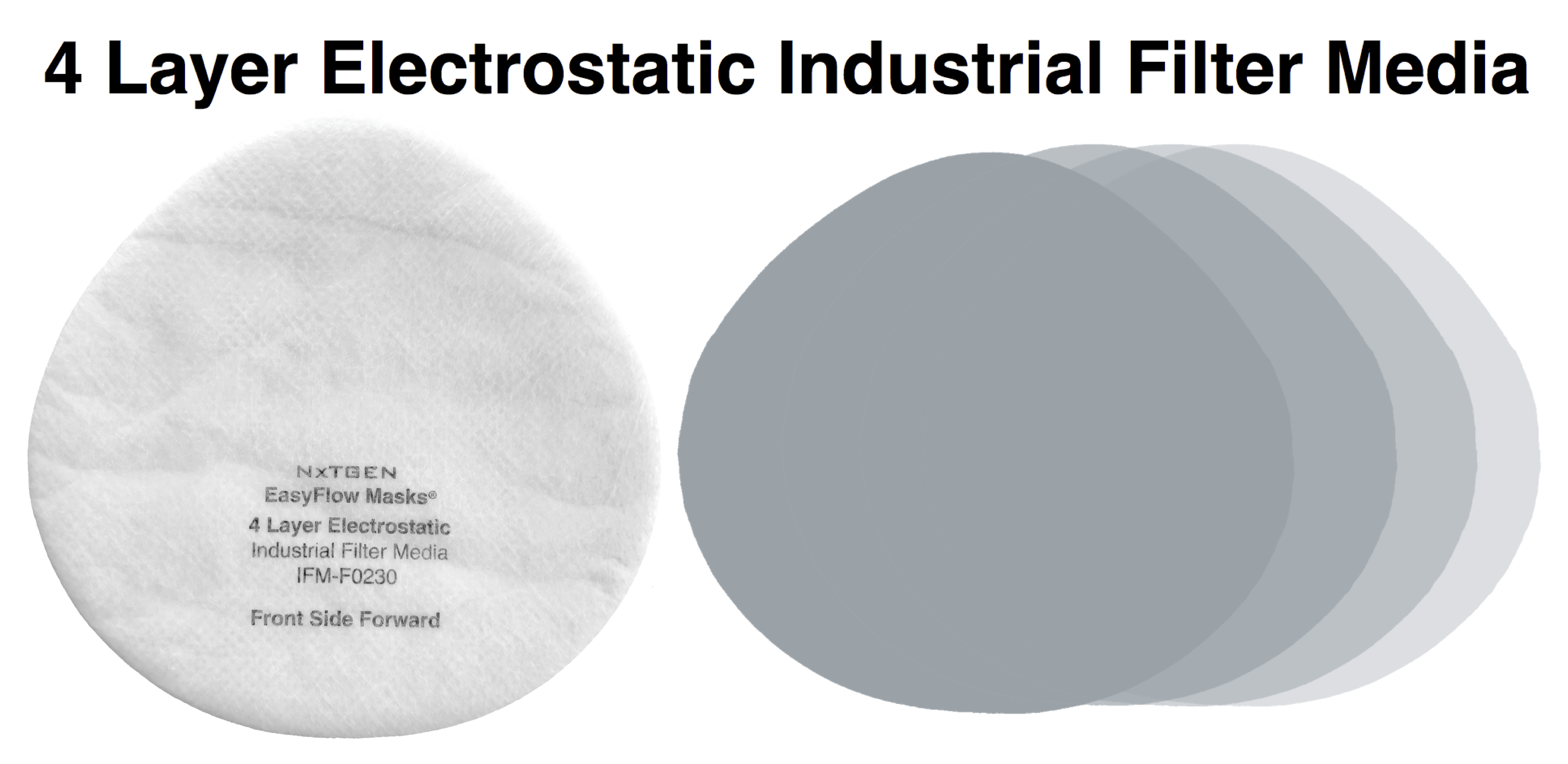
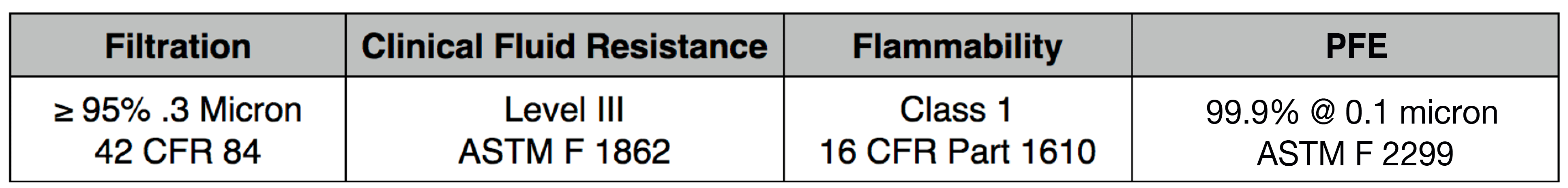
Our 4 layer industrial 95% filter media combines both electrostatic material and meltblown cloth for our highest available level of particle filtration. This electrostatic and meltblown combination provides significant industrial and clinical grade, sub-micron filtration for applications where non-oil particulate respirators are used, as defined by NIOSH 42 CFR part 84.
Recommended usage: non-oil particle filtration including: dust, mist, fumes, fibers, bioaerosols, residual surgical smoke and wildfire pollution. For occupational use, all respirators should be properly fit tested prior to use, per 29 CFR 1910.134, Appendix A.
For clinical use: our 95% filter media meets ASTM Level III fluid resistance and is suitable for surgical, sterile and other environments where medical respirators are required and treatment of patients with known or suspected aerosol transmittable diseases.
International Shipping Available
- Free first class postal to the USA, PR, AK & HI (USA ONLY)
- US Expedited Priority Shipping $9.95 (USA ONLY)
- US 2 Day Air Shipping $20 (USA ONLY)
- Canada Postal $12.00 USD (Canada ONLY)
- Canada Express Shipping $25 USD (Canada ONLY)
- International Express Shipping via DHL $40 USD Flat Rate (All Other International Orders Must Select)
Free basic shipping available to the USA ONLY
All other locations must select the appropriate shipping method based on the above chart, or your order will be canceled. Shipping rates DO NOT include duty, VAT or other import fees, which your local municipality may collect upon arrival.
Shop mask sets by size, color and filter media type
-
Black Replacement Gasket (M-L Size)
$7.50Successfully Added to your Shopping CartAdding to Cart... -
White Replacement Gasket (M-L Size)
$7.50Successfully Added to your Shopping CartAdding to Cart... -
ASTM Level 1 Consumer | Clinical filter Set (5 qty) Valved
$9.95Successfully Added to your Shopping CartAdding to Cart... -
ASTM Level 3 Clinical | Consumer filter Set (5 qty)
$12.95Successfully Added to your Shopping CartAdding to Cart... -
Industrial | Clinical | Consumer 95% Filter Set (5 qty)
$15.95OUT OF STOCKSuccessfully Added to your Shopping CartAdding to Cart... -
ASTM Level 1 Consumer | Clinical filter Set (10 qty) Valved
$16.95Successfully Added to your Shopping CartAdding to Cart... -
ASTM Level 3 Clinical | Consumer filter Set (10 qty)
$22.50Successfully Added to your Shopping CartAdding to Cart... -
Industrial | Clinical | Consumer 95% Filter Set (10 qty)
$27.00OUT OF STOCKSuccessfully Added to your Shopping CartAdding to Cart... -
ASTM Level 1 Consumer | Clinical filter Set (25 qty) Valved
$39.65Successfully Added to your Shopping CartAdding to Cart... -
ASTM Level 1 Consumer | Clinical Set (M-L Size) Valved
$39.95Successfully Added to your Shopping CartAdding to Cart... -
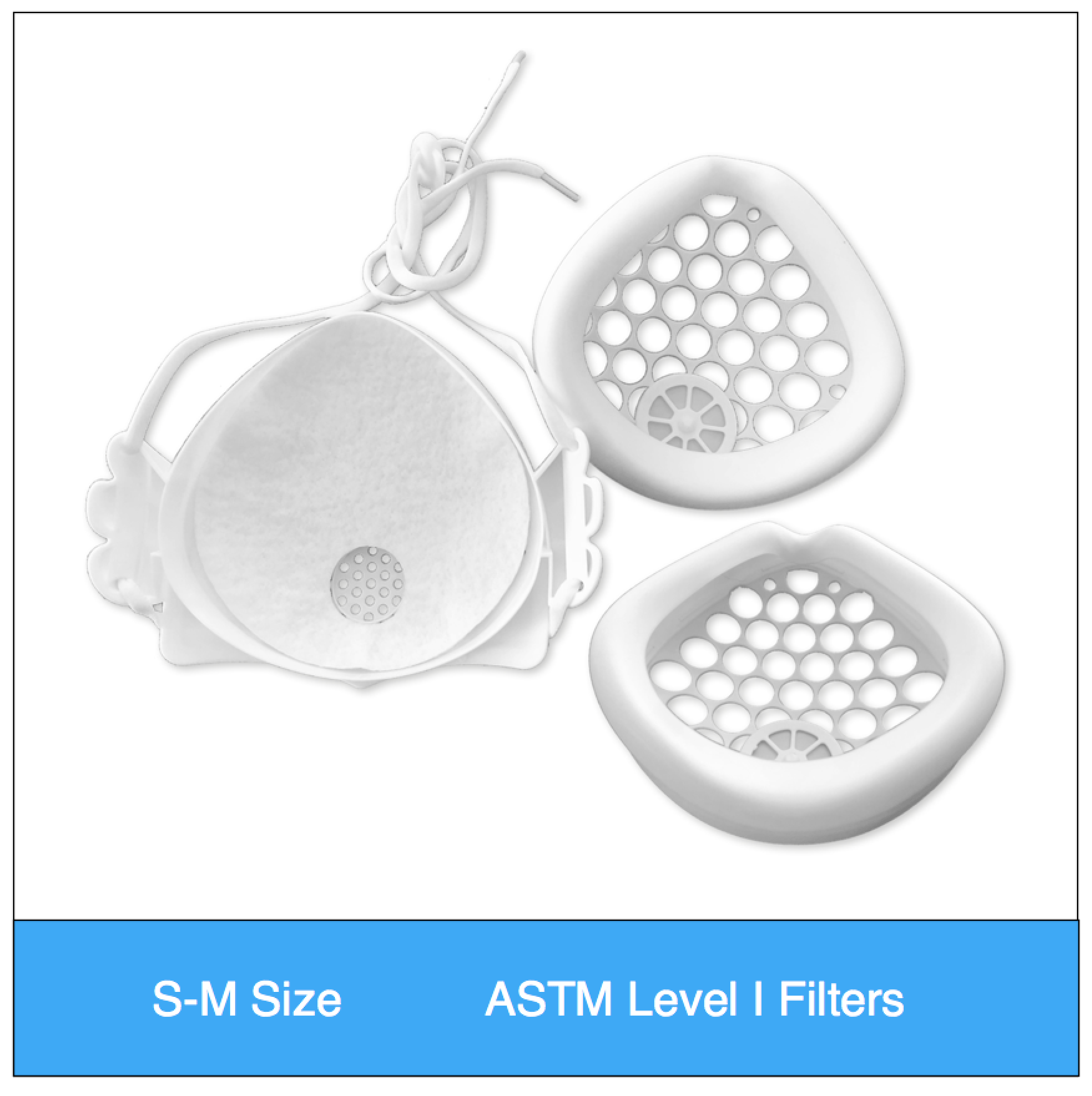
ASTM Level 1 Consumer | Clinical Set (S-M Size) Valved
$39.95Successfully Added to your Shopping CartAdding to Cart... -
ASTM Level 1 Consumer | Clinical Set (M-L Size) Valved
$39.95Successfully Added to your Shopping CartAdding to Cart... -
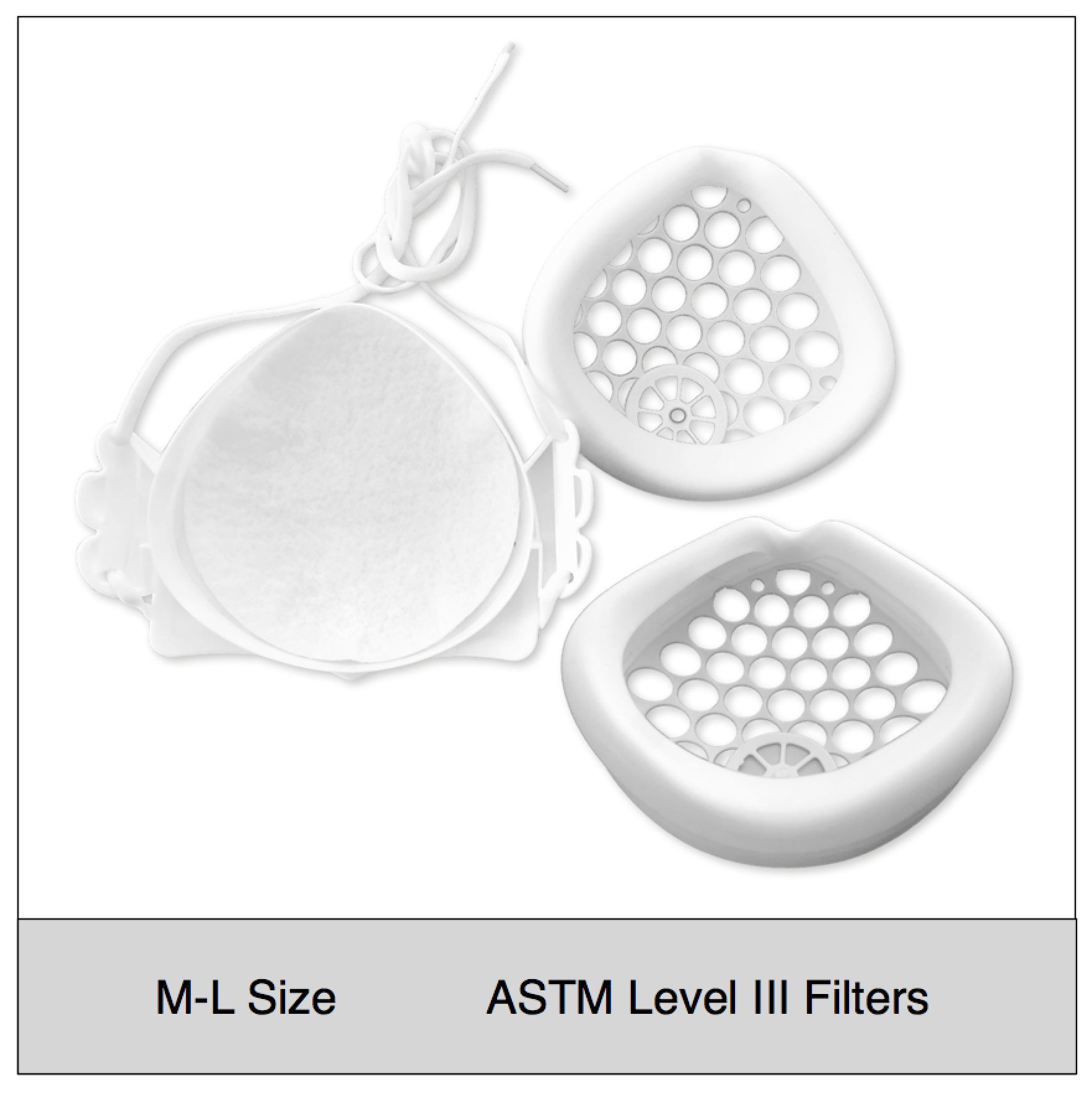
ASTM Level 3 Clinical | Consumer Set (M-L Size)
$39.95Successfully Added to your Shopping CartAdding to Cart... -
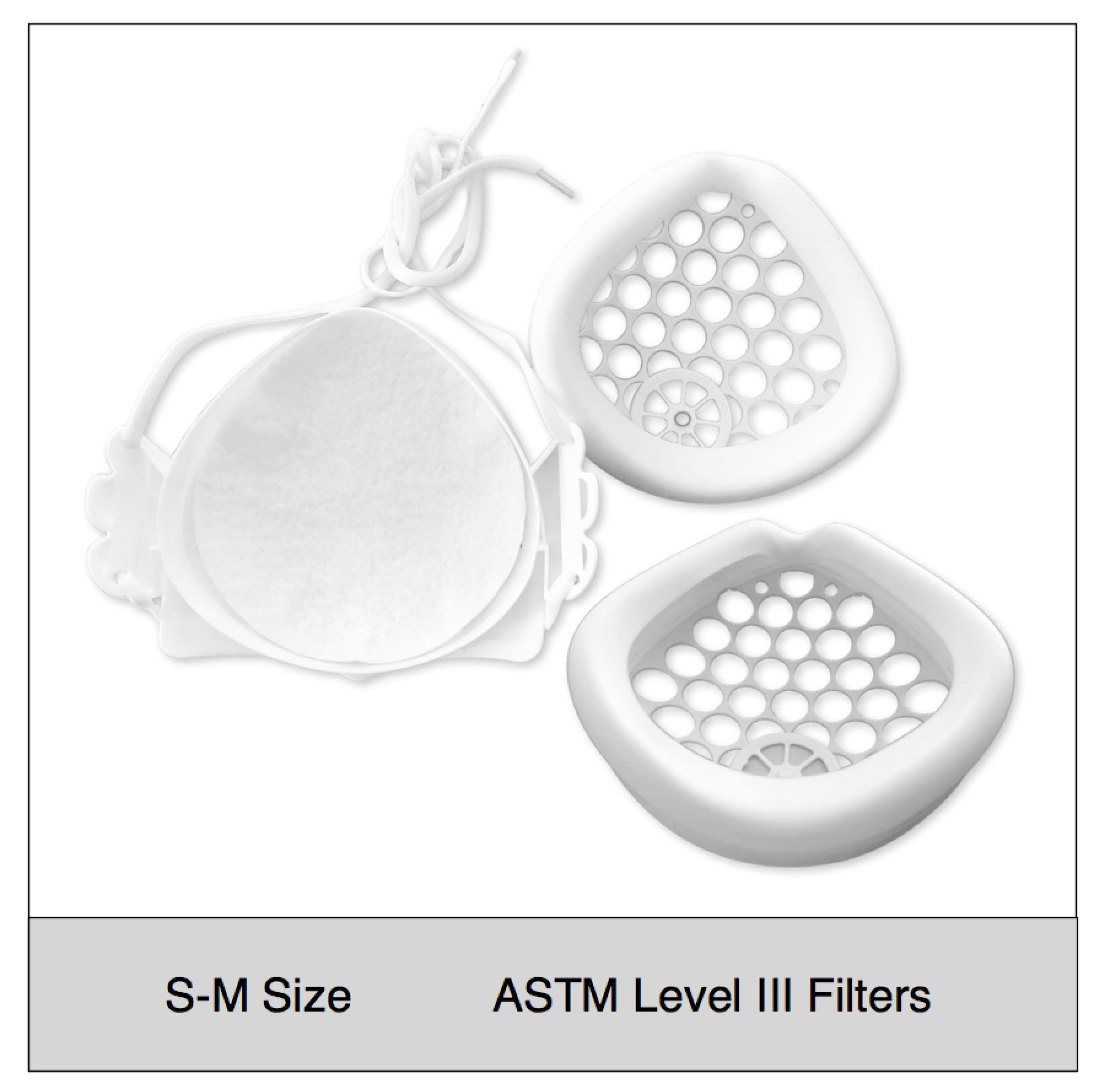
ASTM Level 3 Clinical | Consumer Set (S-M Size)
$39.95Successfully Added to your Shopping CartAdding to Cart... -
Industrial 95% Filter Set (S-M Size)
$39.95OUT OF STOCKSuccessfully Added to your Shopping CartAdding to Cart... -
Industrial 95% Filter Set (M-L Size)
$39.95OUT OF STOCKSuccessfully Added to your Shopping CartAdding to Cart... -
ASTM Level 1 Consumer | Clinical Set (S-M Size) Valved
$39.95Successfully Added to your Shopping CartAdding to Cart... -
ASTM Level 3 Clinical | Consumer Set (M-L Size)
$39.95Successfully Added to your Shopping CartAdding to Cart... -
ASTM Level 3 Clinical | Consumer Set (S-M Size)
$39.95Successfully Added to your Shopping CartAdding to Cart... -
Industrial 95% Filter Set (S-M Size)
$39.95OUT OF STOCKSuccessfully Added to your Shopping CartAdding to Cart... -
Industrial 95% Filter Set (M-L Size)
$39.95OUT OF STOCKSuccessfully Added to your Shopping CartAdding to Cart... -
ASTM Level 3 Clinical | Consumer filter Set (25 qty)
$45.00Successfully Added to your Shopping CartAdding to Cart... -
Industrial | Clinical | Consumer 95% Filter Set (25 qty)
$54.00OUT OF STOCKSuccessfully Added to your Shopping CartAdding to Cart... -
ASTM Level 1 Consumer | Clinical filter Set (50 qty) Valved
$74.00Successfully Added to your Shopping CartAdding to Cart... -
ASTM Level 3 Clinical | Consumer filter Set (50 qty)
$87.00Successfully Added to your Shopping CartAdding to Cart... -
Industrial | Clinical | Consumer 95% Filter Set (50 qty)
$106.00OUT OF STOCKSuccessfully Added to your Shopping CartAdding to Cart... -
ASTM Level 1 Consumer | Clinical filter Set (100 qty) Valved
$138.00Successfully Added to your Shopping CartAdding to Cart... -
ASTM Level 3 Clinical | Consumer filter Set (100 qty)
$169.00Successfully Added to your Shopping CartAdding to Cart... -
Industrial | Clinical | Consumer 95% Filter Set (100 qty)
$207.00OUT OF STOCKSuccessfully Added to your Shopping CartAdding to Cart... -
ASTM Level 1 Consumer | Clinical filter Set (200 qty) Valved
$248.00Successfully Added to your Shopping CartAdding to Cart... -
ASTM Level 3 Clinical | Consumer filter Set (200 qty)
$300.00Successfully Added to your Shopping CartAdding to Cart... -
Industrial | Clinical | Consumer 95% Filter Set (200 qty)
$365.00OUT OF STOCKSuccessfully Added to your Shopping CartAdding to Cart...
Disclaimer: As a new product, the NXTGEN EasyFlow Mask® has not been formally NIOSH or FDA listed yet. This is a lengthy process that often takes a few years to complete. All of our products have been throughly tested and pre-certified by independent, accredited labs. Our medical grade filters meet FDA GMP standards and have completed all ASTM testing for clinical use as listed in the specifications. Our industrial filter media meets and exceeds N95 standards based on NIOSH NaCl testing, per 42 CFR 84. While we cannot yet legally market our clinical grade filters as FDA approved and our industrial grade filters as N95 until this process is complete, our products meet and exceed all FDA, NIOSH and EN standards for use as listed in each filter specification. With proper seal, all NXTGEN masks will pass a qualitative fit test. All of our filter media is made in the USA and processed in an ISO 9001:2015 facility that exclusively specializes in the processing of medical and industrial filter media. Please contact us with any questions or for testing information.